|
Who is Cristopher Bragg? DR. CRISTOPHER BRAGG Dr. Cristopher Bragg is an Assistant Professor of Neurology at Massachusetts General Hospital and Harvard Medical School. His lab studies cellular mechanisms underlying hereditary dystonias, with a current focus on three forms in particular: DYT1 dystonia, DYT6 dystonia, and X-Linked Dystonia-Parkinsonism (XDP). He also serves as the Director of the MGH Collaborative Center for X-Linked Dystonia-Parkinsonism (CCXDP), an international consortium focused exclusively on a rare syndrome that is endemic to the Philippines. What are DYT1, DYT6, and XDP? THAP1 DYT-6 MOLECULAR STRUCTURE DYT1 and DYT6 are both generalized dystonias that typically develop in childhood or adolescence. They are often designated as “isolated” or “pure” dystonias, because in most patients, dystonia is the sole or primary symptom. XDP, in contrast, usually arises in adulthood, most often during the third or fourth decade of life. Unlike DYT1 and DYT6, XDP combines features of both dystonia and parkinsonism often in a temporal progression. The pattern that has been documented most frequently consists of an initial focal dystonia that spreads to other body regions— as parkinsonian symptoms also emerge over time. Some patients, however, may exhibit parkinsonian signs as the first symptom and experience a milder course of disease progression,— and the reasons for this heterogeneity are not yet understood. What causes these disorders? XANDRA BREAKEFIELD PHD The genetic cause of DYT1 was discovered in 1997 by Drs. Xandra Breakefield and Laurie Ozelius at Massachusetts General Hospital. It was the culmination of many years of painstaking work that took place long before the human genome was sequenced, when the methods for finding disease-related genes were still in their infancy. They found that DYT1 patients had a specific deletion in a small stretch of the TOR1A gene, which encodes a protein termed torsinA. Since that first report, a small number of additional mutations in TOR1A have been found, but the vast majority of DYT1 patients all appear to share the same original gene variant. LAURIE OZELIUS PHD DYT6 dystonia is associated with mutations in the THAP1 gene. Unlike DYT1, which is caused by the same gene deletion in nearly all cases, DYT6 has been linked to a large number of different mutations in THAP1. It is not yet known if these different mutations account for differences in symptoms, as so far there are no clear relationships between variants in THAP1 and patterns of clinical disease. While cases of DYT1 and DYT6 have been found in multiple patient populations around the world, XDP has arisen uniquely among individuals with ancestry to the island of Panay in the Philippines. It is caused by the insertion of a long stretch of DNA called a “retrotransposon.” Retrotransposons come in several varieties, many of which have been derived from ancient viruses that deposited their genetic content into our genomes millions of years ago. They are considered “mobile DNA” because of their ability to move from one chromosome to another by reinserting copies of themselves into other genomic locations - a discovery first made by Dr. Barbara McClintock for which she was awarded the Nobel Prize in Medicine in 1983. All living organisms contain various forms of mobile DNA. In humans, these elements are scattered throughout our genomes; most are completely benign and have lost their ability to move over the course of evolution. However, a few types of retrotransposons are still active, and within the Panay population, one such example has become inserted into a gene called TAF1, thereby disrupting its function and causing XDP. BREAKEFIELD LABORATORY Why study these syndromes together? XDP DYSTONIA WHICH IS VERY COMMON ON THE ISLAND OF PANAY Despite the differences between DYT1, DYT6, and XDP, the proteins that are affected in these syndromes play inter-related roles within cells. The DYT6 protein, THAP1, and XDP protein, TAF1, both reside within the cell’s nucleus and regulate how genes are turned on or off, a process known as transcription. The DYT1 protein, torsinA, acts within the adjacent envelope that surrounds the nucleus and is important for its shape and organization. Numerous research studies over many years have characterized how torsinA functions within the nuclear envelope and how the mutation that causes DYT1 dystonia may compromise this cellular compartment. Less is known about THAP1 and TAF1, but recent studies are now revealing how transcription in DYT6 and XDP cells is affected by the dystonia-related variants in these genes.
The Bragg lab studies these and other dystonic syndromes together by analyzing patient cells and characterizing their patterns of gene transcription, which can reveal cellular pathways that are affected in these cells. The overall goal of this work is to look for any defects that may be common to multiple forms of dystonia. If such deficits exist, then they may represent potential targets for designing new therapies that would be of broad benefit to patients with dystonia. Dr. Edgar Rodriguez weighs in on Gene Therapy for Brain Diseases: A Path for DYT-1 Dystonia6/3/2020
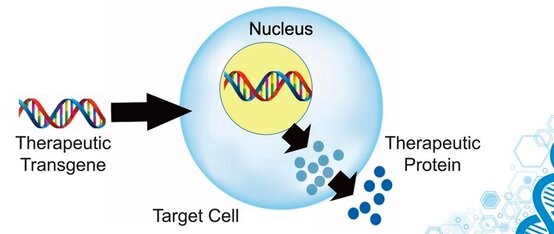
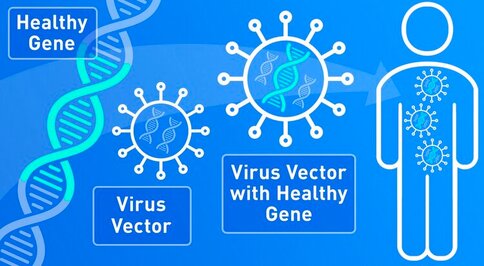 SCIWORTHY.COM (PICTURE) WHICH HAS GREAT IMAGES AND A GREAT SUMMARY WEBSITE OF THIS TOPIC! Gene Therapy for DYT-1 Dystonia and other diseases? We sat down with one the experts on gene therapy for DYT-1 dystonia and other central nervous system diseases and we picked his brain on this topic. The interview was fascinating! Who is Edgar Rodriguez? Dr. Edgardo (Edgar) Rodríguez-Lebrón has 20 years of experience in the gene therapy space with a focus on diseases of the nervous system. He has engineered and developed gene-based therapies to prevent or reverse neurodegenerative processes caused by the accumulation of toxic mutant proteins. He is currently Scientific Advisor to Tyler's Hope, Chief Science Officer of Andante Biologics and a co-founder of early-stage biotech companies developing molecular therapies for CNS diseases. He holds an Adjunct Faculty appointment at the University of Florida where he trained as a graduate student. Go Gators! Often, diseases that affect the brain are caused by genetic mutations that prevent the normal function of a gene. In other cases, genetic mutations can cause a gene to ‘gain’ a new function that is toxic to the brain. Gene therapies are a class of medicines designed to specifically (i) replace a missing genetic function, (ii) block a ‘gained’ toxic genetic function, or (iii) limit or enhance cellular pathways to support overall brain function. What is a viral-based gene therapy?
What makes AAV a desirable vector for CNS-targeted gene therapies?
What are some recent major developments in AAV CNS gene therapy? Recent developments include:
What are some of the major hurdles still being faced by AAV-based gene therapies? Hurdles include:
How can an AAV-based gene therapy be used in DYT1 dystonia? Applications to DYT-1:
What is on the horizon for AAV-based gene therapies as it applies to DYT1? On the horizon:
|
Archives
September 2021
Categories |
Location:
|














 RSS Feed
RSS Feed